Which Pair Will Produce a Buffer Solution
010 mol L HCl and 020 mol L-I NH3 OC. So we will form the country based by adding a strong acid.
Equal volumes of the following pairs of solutions are mixed.
. A steady rmpH is required for the proper functioning of many chemical and biological systems including our blood for reactions to take place. 010 mol L-1 HCl and 005 mol L-1 NH3 D. Equal volumes of the following pairs of solutions are mixed.
Which pair will produce a buffer solution Which one of the following pairs of 0100 mol L-1. This buffer protects against quantities of acid or base as large as 01 moles per liter. So when we add HCL two HF um the contract it uh acid country base of H F F minus will form.
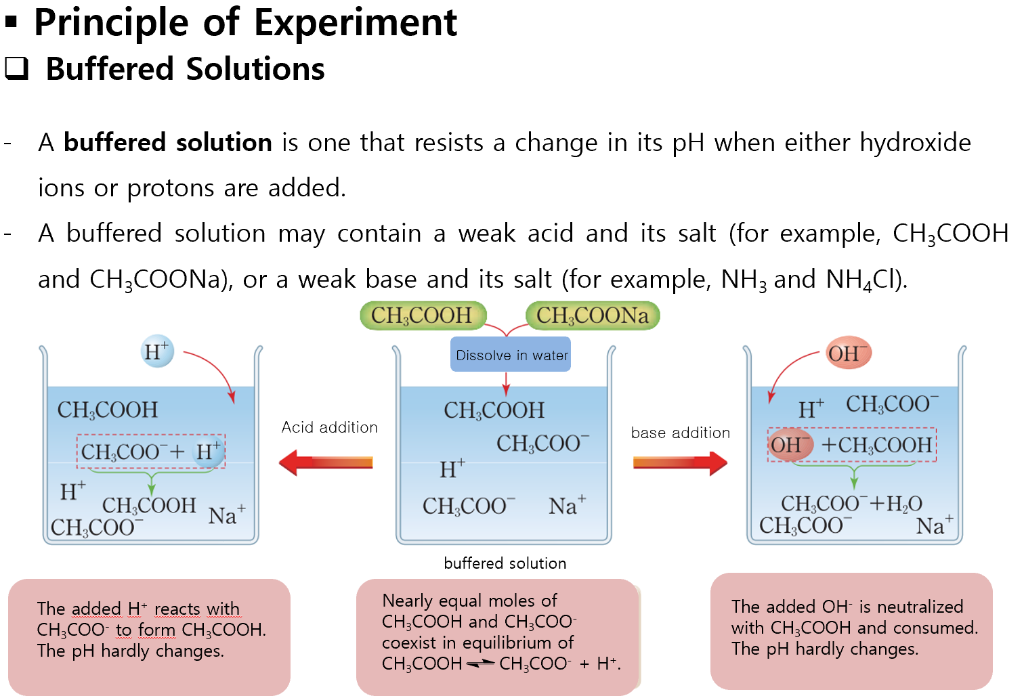
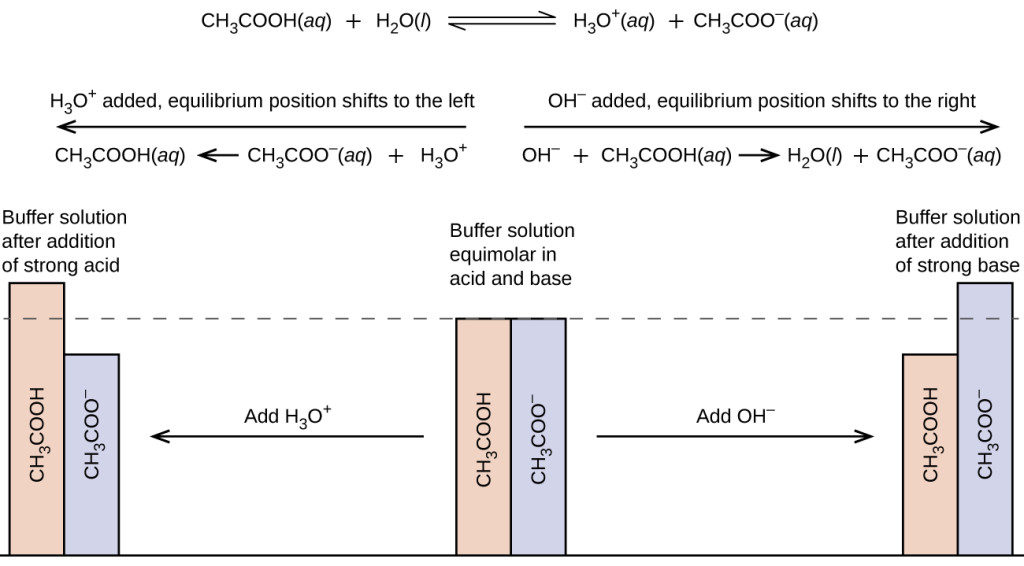
If acid is added the base component of the conjugate pair reacts to form the conjugate acid. 010 mol L HC1 and 005 mol L- NaOH 035 mol L CH3COOH and 025 mol L NaOH 010 mol L-HC1 and 005 mol L NH. The pH of blood is controlled by the buffering action of several conjugate acid-base pairs.
A is not a good buffer because sulfuric acid is not a weak acid. Hemoglobin a protein is the red substance in the blood. Acidic buffer- This is formed by a weak acid and its salt of strong base.
Which of the following pairs would make a good buffer solution in an aqueous solution. A buffer of carbonic acid H 2 CO 3 and bicarbonate HCO 3 is needed in blood plasma to maintain a pH between 735 and 745. 010 mol L - HCl and 020 mol L-1 CH3COOH O e.
A H2SO4 and NaHSO4 B Ca NO32 and HNO3 C HCl and NaCl D HF and NaOH E none of them I know the answer is D but I dont understand why. So if we have a conjugate acid based pair we will be able to form a buffer. 010 mol L-1 HC1 and 020 mol L CH3COOH 010 mol L- HC1 and 020 mol L- Naci.
You are also correct that a good buffer is a weak acid or weak base and its conjugate baseacid. Thus the resultant solution contains N H 4 C l and N H 4 O H which will produce a buffer solution. A solution with appreciable concentrations of both members of a conjugate pair is known as a buffer.
By definition the buffer capacity of a buffer is the amount of acid or base that can be added before the pH of the solution changes significantly. 010 mol L-1 HCl and 020 mol L-1 CH3COOH E. Therefore it along with sodium chloride is not a good buffer.
The mechanism involves a buffer A solution that resists dramatic changes in pH a solution that resists dramatic changes in pH. Are strong acids and hence they cannot be used as buffers. Among the following compounds NaOH is a strong base and HCl andHNO 3.
So for B we dont have this but we need to look closer. HA aq H 2 O l -- H 3 O aq A - aq K a H 3 O A - HA A buffer system can be made by mixing a soluble compound that contains the conjugate base with a solution of the acid such as sodium acetate with acetic acid or ammonia with ammonium chloride. This resistance is created by having both members of the conjugate pair.
Part B HCl and NaOH dont form a buffer in aqueous Solution. D is a good buffer because it is a weak acid and its salt. Buffer solutions achieve their resistance to pH change because of the presence of an equilibrium between the acid and its conjugate base or the base and its conjugate acid.
010 mol L - HCl and 020 mol L - NaCI. Part C NaOH and NaCN will not form a buffer in aqueous. 010 mol L-1 HCl and 015 mol L-1 NH3 C.
Memorize flashcards and build a practice test to quiz yourself before your exam. Start studying the Chem 210 Chapter 19 flashcards containing study terms like Which of the following aqueous mixtures would be a buffer system Equal volumes of the following pairs of solutions are mixed. Buffers do so by being composed of certain pairs of solutes.
Buffer solutions are of 2 types. Which of the following will produce a buffer solution when mixed in equal volumes. NAWhich of the following will produce a buffer solution when mixed in equal volumes.
The most important of these is undoubtedly the H 2 CO 3 HCO 3 pair but side chains of the amino acid histidine in the hemoglobin molecule also play a part. Basic buffer- This is formed by a weak base and its salt of strong acid. Which pair will produce a buffer solution.
Buffers are solutions that resist changes in pH. Industrially buffer solutions are used in fermentation processes and in setting the correct conditions for dyes used in coloring fabrics. D is the correct answer.
Part A HCN and NaCN form a buffer in aqueous Solution. Which pair will produce a buffer solution. If base is added the acid component of the conjugate.
A buffer solution can be made by mixing a weak acid with one of its salts OR mixing a weak base with one of its salts. 010 mol L- HCl and 005 mol L -1 NH3 d. Note that the capacity of the second buffer solution in the table above is even greater than the first.
Incorrect Question 8 0 413 pts Equal volumes of the following pairs of solutions are mixed. A more technical way of saying this is that a buffer solution consists of a mixture of a weak acid and its conjugate base OR a. Which pair of solutions forms a buffer solution when equal volumes of each are mixed.
010 mol. However HCl is not a weak acid. Either a weak acid plus a salt derived from that weak acid or a.
010 mol L-1 HCl and 005 mol L-1 NaOH B. NAWhich of the following will produce a buffer solution when mixed in equal volumes Books. Buffers are required in such systems in order to maintain a consistent rmpH An aqueous solution comprising a weak acid and its conjugate base or a weak base and its conjugate acid is.
Which pair will produce a buffer solution. A 1 mol dm-3 NH 4 OH and 01 mol dm-3 HCl b 005 mol dm-3 NH 4 OH and 01 mol dm-3 HCl c 1 mol dm-3 NH 4 OH and 005 mol dm-3 HCl d 1. 010 mol L-1 HCl and 020 mol L-1 NaCl.

Solved Preparation Of Buffer Solution Experiment I Uploaded Chegg Com


No comments for "Which Pair Will Produce a Buffer Solution"
Post a Comment